Ancillary Studies
HeartShare seeks to better understand the biological basis of heart failure with preserved ejection fraction (HFpEF) by using cutting edge technologies, big data, and artificial intelligence, with the ultimate goal of finding new ways to prevent and treat HFpEF. We welcome innovative and rigorous ancillary studies that will enhance HeartShare and lead to a better understanding of heart failure and related conditions. The HeartShare ancillary studies process is overseen by the Publication and Ancillary Studies (PAS) Committee.
Policies
The official policy for ancillary studies, approved by the PAS Committee on September 21, 2023, are available here.
Submitting an Ancillary Study Proposal
To submit an ancillary study proposal for review by the PAS Committee, investigators must fill out the form here: https://redcap.link/z4424151. The PAS Committee will review the proposal and provide the investigator with feedback to help strengthen the proposal. Once an ancillary proposal is approved by the PAS Committee, the HeartShare DTC will provide an official Letter of Support to the investigator to accompany their submission.
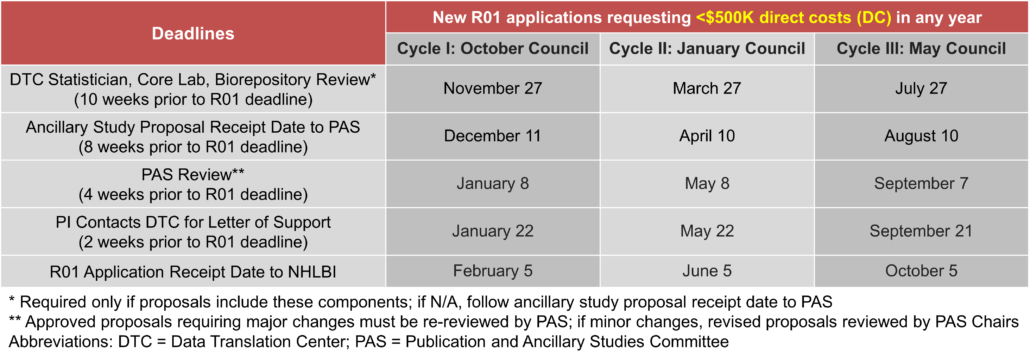
Deadlines
Ancillary studies require proposal submission 8-10 weeks prior to grant deadline depending on the requested budget (see deadline tables below for specifics). Note that earlier deadlines apply for applications requesting >$500K direct costs in any year due to the NHLBI 500K Process. Ancillary studies involving a subcontract must have their final budget negotiated and approved for review with the DTC and each clinical center no later than 5 weeks prior to a funding application.
Questions?
More guidance is available in this slide deck from the NHLBI. If you have additional questions, please reach out to HeartShareStudy@northwestern.edu!
