Study Design
HeartShare is comprised of retrospective and prospective components.
The HeartShare Retrospective study will aggregate and harmonize data from previously completed cohort studies and trials. The phenotype data will be combined with an image repository and additional omics assays.
The HeartShare Deep Phenotyping Study is composed of 2 prospective, multicenter, longitudinal components: (1) the HeartShare Registry and (2) the HeartShare Deep Phenotyping Cohort study involving up to 10,000 and 2,000 participants, respectively, enrolled from at least 6 HeartShare Clinical Centers.
Together the retrospective and prospective cohorts will create a rich repository of data (e.g., demographic, social determinants of health, clinical, physiological, laboratory), images, and multi-omics (blood and tissue samples) that will serve as a resource for investigators to identify novel HFpEF subtypes and mechanisms (biological and pathophysiological) to inform future strategies for enhanced diagnosis and treatment of HFpEF.
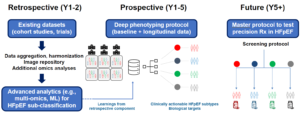
Data Collection at the HeartShare Clinical Centers
There are 3 levels of data collection at the 6 HeartShare clinical centers. Level 1 involves retrospective and prospective de-identified EHR data and image collection of all living heart failure patients and age- and sex-matched compartors who have had contact with the health system at each clinical center from 2016 onwards. Level 2 is a prospective “low touch” registry that is a subset of level 1. Patients identified via the EHR query are invited to participate in HeartShare using the Eureka app, supplemented by outreach by clinical center staff to include underserved populations who may not have access to mobile phones or computers. Level 3, the prospective deep phenotyping study, is a subset of level 2 and is recruiting 1000 participants (750 HFpEF, 250 non-HFpEF).
Eureka Platform
HeartShare uses the Eureka platform for participant engagement. Eureka is a cross-platform (iOS, Android, web) tool that provides the following services: eConsent, patient messaging, survey tools, eVisits, mHealth integration, outcomes assessment, EHR integration, study management, and geo-tracking for hospitalizations. The Eureka platform has been used extensively and validated in older participants with chronic medical conditions, including nearly 65,000 participants age > 65 years across all prior Eureka studies. For more information, visit: https://info.eurekaplatform.org
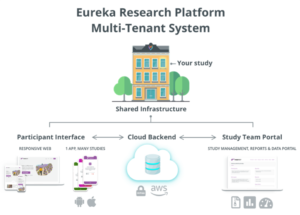
Patient Engagement: We will leverage the Eureka platform to create an interface for patient participants in the prospective component of HeartShare that allows eConsents and completion of forms and that facilitates communication with participants; collects mobile health (mHealth) data from home devices, sensors, and mobile apps; and seamlessly integrate with other data sources in BioData Catalyst. Eureka will allow HeartShare patients to engage in all phenotyping protocols. The Eureka platform, developed at UCSF with NIH-funding (U2CEB021881), is a direct-to-participant digital platform as a resource for enabling efficient mHealth research. Detailed information about the Eureka Research Platform can be found at https://info.eurekaplatform.org. Building upon experience and technology developed by the UCSF team for the Health eHeart Study, >55 individual research studies have been launched on the Eureka platform, engaging over 500,000 participants and led by researchers at multiple institutions across multiple disease areas. The Eureka Platform, which is available on the web and Eureka’s native mobile apps (iOS and Android), is designed for scalability, cost-effectiveness and speed of study startup. The platform is architected with a robust backend that can host numerous studies, each with its own unique look and feel, study design, study activities and flow (Eureka Platform in HeartShare Figure). The backend dynamically delivers content to a Eureka mobile app and/or web-based interface. The Eureka architecture will allow for the rapid (and very cost-efficient) development of a HeartShare mHealth tracking and patient engagement resource without the need to develop a new app de novo. Eureka can be developed and deployed in 3-6 weeks. Eureka is a custom-built, HIPAA-compliant platform that runs on AWS, allowing scalability, and provides for redundancy, security, and data integrity. While Eureka is optimized for remote study participation, it is also equipped for in-person studies and hybrid studies with both remote and in-person components. Built as a national collaborative resource for mHealth research, Eureka is currently used for projects funded by NIH, PCORI, industry and others, including several large multi-center studies. The table below displays the many functions provided by the Eureka platform, which are relevant to HeartShare.
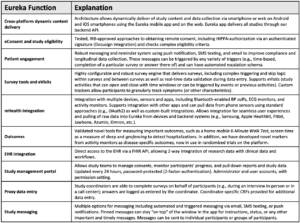
Through the Eureka Study Management Portal, HeartShare personnel can export CSV-formatted files that are automatically updated overnight and thus up-to-date every 24 hours. For larger files (for example raw data from integrations), files (typically JSON formatted) will be uploaded and stored in an AWS S3 bucket for easy programmatic access HeartShare staff. Eureka data has been used for several AI and “big data” studies. Eureka is also supported by a robust bio-signal processing platform using the AMPS CER-S software on a large server to ingest static ECG, continuous ambulatory ECG monitoring, continuous photoplethysmography (PPG) data, and other signals from wearables. The software has robust processing, enables custom algorithms, and has a system for adjudicating (or labeling) these signals.
For HeartShare participants who do not have access to internet or technology, and therefore cannot use the Eureka web- or smartphone-based apps, we will follow the ADAPTABLE clinical trial protocol and offer a non-internet enrollment pathway and survey completion via HeartShare Clinical Center staff or the DTC call center staff, which will involve telephone follow-up with study coordinator data entry into Eureka directly.
Patient Experience: Interested participants, patients with heart failure with preserved ejection fraction (HFpEF) and comparators without HFpEF, will be invited to sign up for Eureka on the iOS or Android mobile app or on the study website. They will create an account with an embedded Unique Participant Code that will be linked the patient’s MRN. Upon account creation, they will be presented with an eligibility survey, and eligible participants will be prompted to sign the study eConsent. The Eureka platform will deliver surveys to the participant on a predetermined cadence. Each “batch” of surveys is called an eVisit, and these eVisits will be open for one month (except the baseline eVisit which will be open for 3 months).
The Eureka platform will send regular reminders via Push Messages, SMS Messages, and emails to remind participants of their incomplete surveys. Once eVisit surveys are completed, the participant will no longer receive reminders until their next eVisit opens.

 Accelerating Medicines Partnership (AMP) Heart Failure
Accelerating Medicines Partnership (AMP) Heart Failure